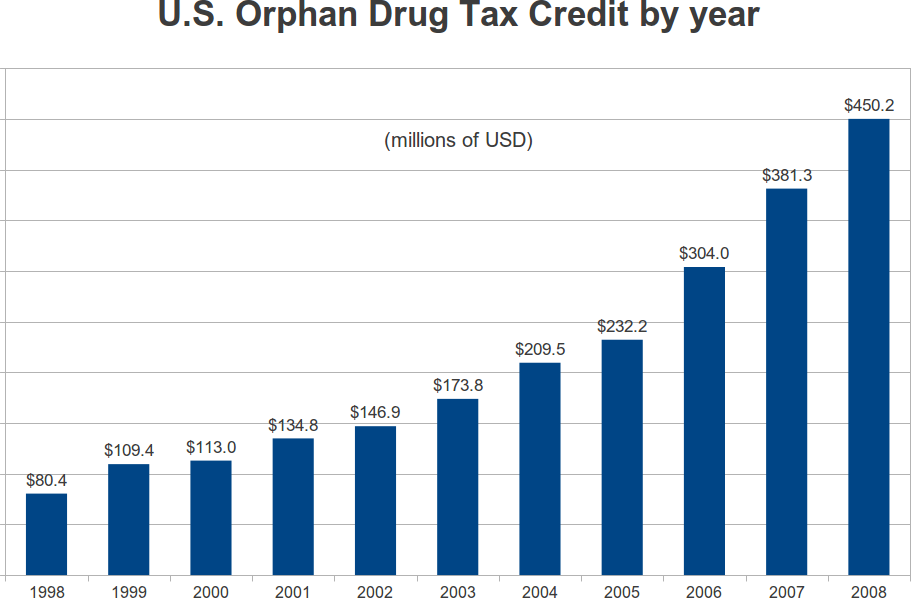
The Orphan Drug Tax Credit, from 1998 to 2008
In 1997, the Orphan Drug Tax Credit was made permanent. In 1998, $80.4 million in credits were claimed on tax returns. By 2008, the claimed credits totaled $450.2 million. This represents a compound growth rate of 17 percent per year. Over the eleven year period, the IRS reported total credits of $2.3355 billion. This is not much when you consider that the orphan tax credit covers 50 percent of the cost of qualifying clinical trials. During the period from January 1, 1997 to December 31, 2008, the FDA granted 1163 orphan designations for which the credit applied. In the same period, the FDA gave marketing approval to 183 indications. On a per-approval basis, that works out to 2335.5/183 = $12.8 million per indication that received FDA marketing approval.
It is also useful to note that there were 6.4 indications for each one receiving marketing approval — a useful statistic regarding the hazard rate for orphan products.
Does the credit make economic sense? If 50 percent of cost of clinical trials was $12.8 million per indication that received marketing approval, how does this compare to the costs of the higher prices associated with it Orphan Drug Act’s 7 years of exclusivity? Is there a more cost effective way to reward or finance investments in orphan indications?